REPROGRAMMING AN ATP-DRIVEN PROTEIN MACHINE INTO A LIGHT-GATED NANOCAGE
Reprogramming An ATP-Driven Protein Machine Into A Light-Gated Nanocage
Hoersch, D, Roh, S, Chiu, W, Kortemme, T
Nat Nano 8(12):928-32, 2013
Hoersch, D, Roh, S, Chiu, W, Kortemme, T
Nat Nano 8(12):928-32, 2013
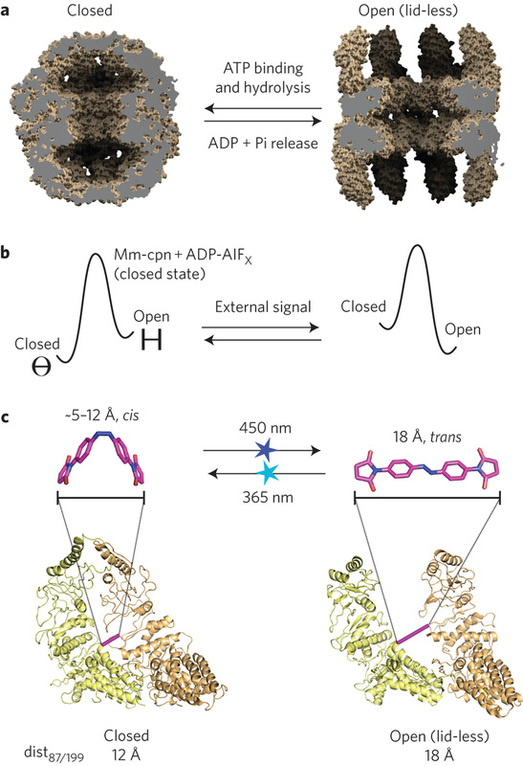
There has been considerable interest in light-based control of biological systems, with successful applications that include light-modulation of neuronal ion channels, light-switchable signaling proteins and light-controlled protein targeting, with examples demonstrated in vitro, in cell culture, and in whole animals. Compared to chemical approaches, light-based methods offer titratable, specific, precise spatial and temporal regulation. Most existing examples of light-based control fall into one of two categories: (a) those that are genetically encoded using a recombinantly-produced protein borrowed from nature (e.g. LOV domains), and (b) those created via targeted insertion of amino acids into a protein sequence and subsequent reaction with them of an exogenously introduced photoisomerizable small molecule, typically azobenzene based. Azobenzene and related molecules undergo a reversible cis–trans isomerization when exposed to specific wavelengths of light, and this change in molecular shape can be coupled to changes in protein function.
Here, we used a type-b strategy to manipulate the conformation of a supramolecular compex. Supramolecular protein assemblies are attractive materials for engineering nanoscale machines with controllable functions. Protein monomers have been engineered to self-assemble into symmetrical complexes, metal-templated structures and cage-like architectures. The mechanistic complexity of protein machines, however, has made it difficult to engineer assemblies with not only new architectures but also desired functions. We applied an azobenzene-based strategy to convert Mm-cpn, an ATP-dependent homo-oligomeric group II chaperonin to a light-driven machine that undergoes large-scale conformational changes between open and closed states, as visualized by single particle cryo-electron microscopy. The resulting light-gated nanocontainer can capture and release non-native cargos. The design principle of alternately stabilizing conformational states by switching atomic distances illuminates the cooperativity of evolved protein assemblies and provides a strategy for engineering other light-controlled biologically inspired machines.
Here, we used a type-b strategy to manipulate the conformation of a supramolecular compex. Supramolecular protein assemblies are attractive materials for engineering nanoscale machines with controllable functions. Protein monomers have been engineered to self-assemble into symmetrical complexes, metal-templated structures and cage-like architectures. The mechanistic complexity of protein machines, however, has made it difficult to engineer assemblies with not only new architectures but also desired functions. We applied an azobenzene-based strategy to convert Mm-cpn, an ATP-dependent homo-oligomeric group II chaperonin to a light-driven machine that undergoes large-scale conformational changes between open and closed states, as visualized by single particle cryo-electron microscopy. The resulting light-gated nanocontainer can capture and release non-native cargos. The design principle of alternately stabilizing conformational states by switching atomic distances illuminates the cooperativity of evolved protein assemblies and provides a strategy for engineering other light-controlled biologically inspired machines.